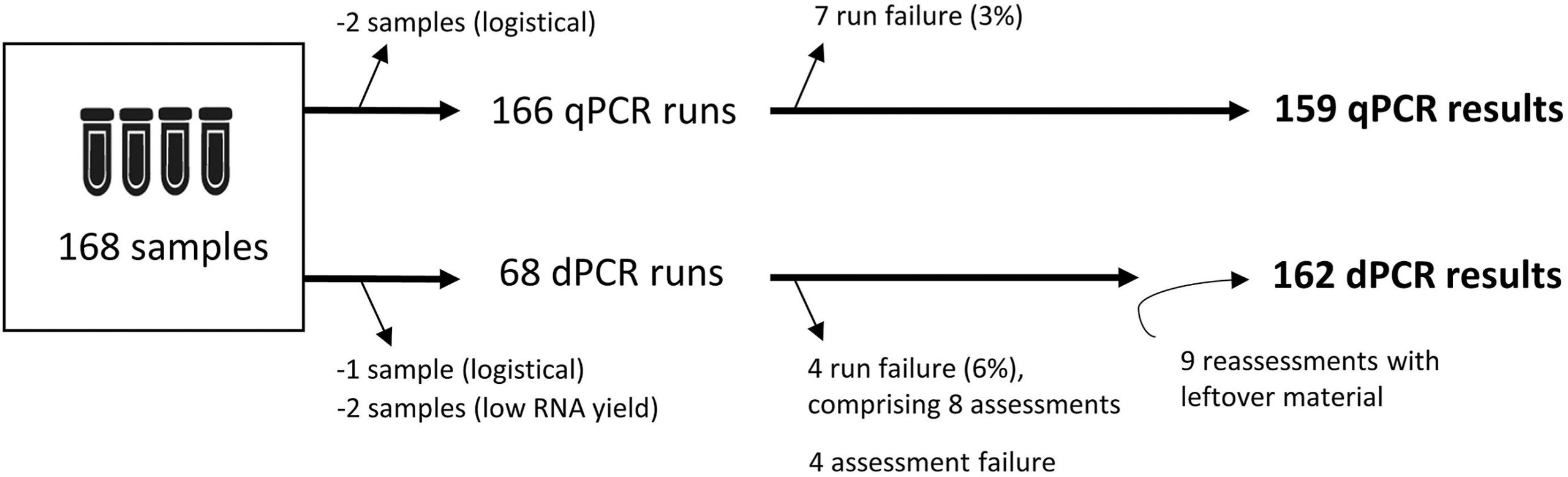
Sample Inclusion and Performed Real-Time Quantitative PCR (QPCR) and Digital PCR (DPCR) Runs. CREDIT: The Journal of Molecular Diagnostics (2024). DOI: 10.1016/J.JMOLDX.2024.11.003
Researchers have found that clinical application of bcr :: ABL1 Digital PCR can reliably quantify stable molecular Remission of Chronic Myeloid Leukemia (CML), Which Will hell to determining for which Pients Chronic Drug Treatment COULD POTENTIALLY BE DISCONTINUED. This transcript that is unique for cml is more sensitive and accurate than the current standard, real-time quantitative PCR (RT-QPCR), for detecting ultlow levels of residual leukemic dissease. Results are reported in The Journal of Molecular Diagnostics.
Lead Investigator Peter E. Westerweel, MD, PH.D., Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, The Netherlands, Says, “In Our Research, We Show That Digital PCR :: ABL Reache A suffgence High Sensitivity in Almost All (97%) Samples of Samples of Patients in Deep Molecular Remission. The molecular Target was detected in Two-Thirds of Patients That Below the Limit of the Standard RT-Qpcr Technique. Remission ELIGIBLE FOR DRUG TREATMENT DISCONTINUATION. “
BCR :: ABL1 is a Specific Genetic fusion protein that is characteristic of cml, Which is Created when Two Genes, BCR and ABL1, Becoma Abnormally Joined Together. With Targeted Therapy Using Tyrosine Kinase Inhibitors (Tkis), Patients with Cml May Achieve Deep Molecular Responses. Patients Who Reach this depth of remunion can have a normal life expectancy and may qualify for a tki discontinuation attempt.
In A Nationwide Prospective Multicenter Study, Samples Were Collected from Dutch Pats With Cml For Whom A TKI Discontinuation Attempt Was Under Consideration. From July 2020, the Total of 168 Samples from 136 Patients with cml from 31 Medical Centers Were Received Until The Time of Analysis In May 2023.
Commenting on the Findings of the Study, Dr. Westerweel says, “BCR :: ABL1 Digital PCR Was Found to Accuity Quantify BCR :: ABL1 Around the level of 0.0023% on the International Scale, Which is the clinically relevant prediction Cutoff Cutoff-free of treatment The Target Sensitivity was set at MR5.0, Requyring Rliable Detection of One Transcript in a Background of At Least 100,000 Regular Copies. Detection of Current Standard RT-QPCR. “
In addition, Researchers Noted That There was the Difference Beteen Patients in the Fluorescence Level of Droplets Rendered by the Digital PCR Technique. This was due to a different in transcript type carried by individual patients. Droplets with Higher Fluorescence Contain The Target Transcript Type and Are Considred Positive WHILE DROLETS with Low Fluorescence Are Considred Negative.
Dr. Westerweel Explains, “Some Patients Have A So -Called E13A2 Transcript Type, While Ourers have an E14A2 Transcript Type. We Validated That the Assay Can Be Used to Identified the Transcript Type In Patients With Detectable Disease. Discovery is very relevant as we have Previously Shown that the transcript type is a molecular for molecular relationship after Drug Discontinuation. Often, The Transcript Type is Not Known for Patients and Cannot Be Established Standard Standard Techniques AT in Deep Remission. “
Digital PCR for BCR :: ABL in this Study Used an FDA-APPROVED COMMERCIALLY AVAILABLE Assay, Which Makes General Use Feasible. This technology may improve the management of cml by enabling more accuise monitoring of minimal residual dissease and better risk assessment for patients considerating treatment-free remission.
Dr. Westerweel Concludes, “Digital PCR for BCR :: ABL is a valuable and Relable Tool to Aid Clinical Decision Making in Cml.”
More information:
Camille Kockerols et al, BCR :: ABL1 Deep Molecular Response Quantification and Transcript Type Identification in Chronic Myeloid Leukemia Using A US Food and Drug Administration-APProved Dropplet-Based Digital PCR Assay, The Journal of Molecular Diagnostics (2024). DOI: 10.1016/J.JMOLDX.2024.11.003
Citation: Digital PCR Identifies Leukemia Patients Who Can Stop Drug Treatment (2025, March 27) Retrieved 27 March 2025
This document is Subject to Copyright. Apart from Any Fair Dealing for the Purpose of Private Study or Research at Part May Be Reproduced Without The Written Permission. The Content is Provided for Information Purposes Only.